Connect with Best Oncologists
Get Free Personalized quote for CAR T Cell Therapy
 |
 |
 |
 |
 |
Highlights
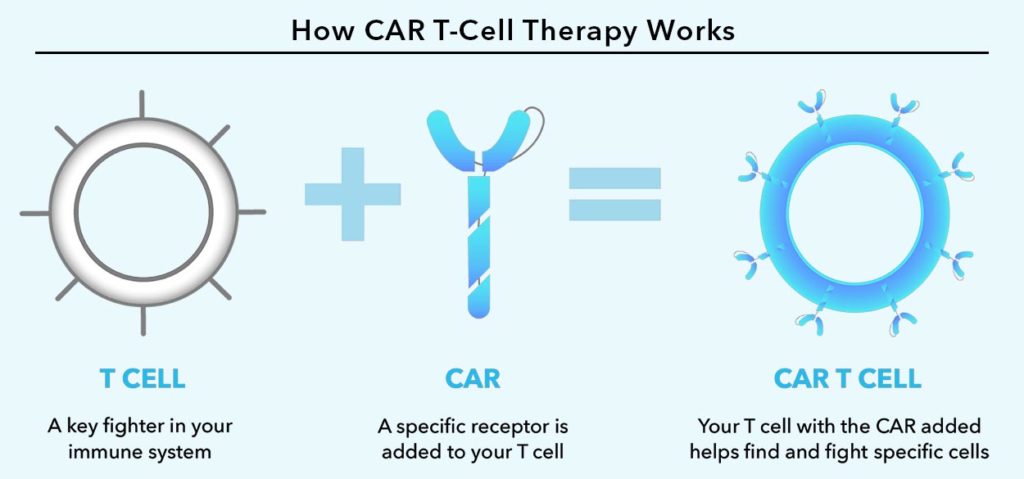
Chimeric Antigen Receptor (CAR) T-cell therapy is an immunotherapy in which the patient’s own T cells are modified to recognize and attack the cancer cells. T cells are a part of our body’s immune system and function as killer cells by destroying the abnormal cells. But, unfortunately, these T cells are unable to recognize the cancer cells or destroy them completely.
In this technique, the T cells are collected from the patient’s blood and are genetically engineered in the laboratory so that they recognize the cancer cells as targets. The cells are altered to produce special receptor on their surface, known as chimeric antigen receptors (CARs).
These receptors have the ability to recognize the specific structure (antigen) present on the surface of the cancer cells. This enables the modified T cells, known as CAR T cells, to become activated and attack the tumour cells in the body. A large number of these CAR T cells are grown in the laboratory so that they are sufficient in number and then infused into the patient’s bloodstream to fight cancer.
Several CAR T-cell therapies have been approved by the US Food and Drug Administration (FDA) for patients with specific blood cancers that are not responsive to chemotherapy or other treatments. Those with blood cancer who have had prior successful treatments but still have the disease can also be treated with this therapy.
Specialized programs known as risk evaluation and mitigation strategies (REMS) are used to provide CAR T-cell therapy. REMS guarantees that medical professionals are qualified to administer the therapy and are skilled in handling any potentially dangerous side effects.
The following details on the cancers currently being treated with CAR T-cell therapy:
Acute lymphoblastic leukemia in B cells (ALL)
White blood cells or immature B lymphocytes are impacted by this cancer while they are developing in your bone marrow. Bone marrow transplants and chemotherapy are the usual treatments used by providers.
FDA-approved CAR-T cell treatments consist of:
Non-Hodgkin lymphoma in B cells
Diffuse large B-cell lymphoma (DLBCL), follicular lymphoma with DLBCL, and high-grade B-cell lymphoma are among the forms of B-cell non-Hodgkin lymphoma. Stem cell transplantation, monoclonal antibodies, and chemotherapy are the usual treatments used by providers for these conditions.
FDA-approved CAR-T cell treatments consist of:
Primary mediastinal large B-cell lymphoma.
FDA-approved CAR-T cell treatments consist of:
Mantle cell lymphoma
This particular kind of non-Hodgkin lymphoma begins in the B cells in your body. Stem cell transplantation and chemotherapy are the usual treatments used by providers.
FDA-approved CAR-T cell treatments consist of:
Multiple Myeloma
This is a cancer of the cells that produce antibodies, called plasma cells. Stem cell transplantation, targeted therapy, or chemotherapy are the methods used by medical professionals to treat this illness.
The following CAR-T cell therapies have FDA approval:
Researchers are examining the potential benefits of CAR T-cell therapy for patients with non-small cell lung, brain, or breast cancers.
Connect with Best Oncologists
Get Free Personalized quote for CAR T Cell Therapy
 |
 |
 |
 |
 |
The T cells are isolated, sent to the lab, and modified by introducing the gene for the particular chimeric antigen receptor (CAR) after the white cells are eliminated. They are therefore CAR T cells. Then, in the lab, these cells are expanded and multiplied. The production of the vast quantity of CAR T cells required for this treatment can take several weeks.
The US Food and Drug Administration (FDA) has authorized CAR T-cell therapies for the treatment of multiple myeloma and certain types of leukemia and lymphomas. Usually, CAR T-cell therapy is used following the failure of other forms of treatment.
Currently approved CAR T-cell therapies include, for example:
To treat other forms of cancer as well, numerous additional CAR T-cell therapies and related forms of treatment are currently being investigated in clinical trials.
CAR T cell therapy is a complex procedure that should only be performed by experts with extensive experience.
Before initiating the therapeutic intervention, the medical practitioner conducts various assessments to determine the suitability of the individual for the procedure. The treatment protocol encompasses a series of sequential steps, which include:
Collection of T cells from the patient
Bio-engineering of the T cells
The T cells collected from the blood are sent to the laboratory where they are genetically altered to express chimeric antigen receptors (CARs) on their surface.
Increasing the number of CAR T cells
Conditioning
Re-infusion of the cells
Most patients require a week to ten days in the hospital for their medical professionals to treat any side effects and keep an eye on how they are responding to the treatment.
The CAR T-cells might be administered to the patient without requiring them to stay in the hospital.
The doctor will continue to keep an eye on the procedure and monitor the advancement if that is the case. One might have to go back to the hospital to finish the procedure for those who experience side effects.
Treatment with CAR T cells may have serious or even fatal side effects. For this reason, patients receiving this treatment are typically required to stay in the hospital for a few days for medical professionals to monitor and treat any side effects.
One should arrange to have someone with them around the clock during the first month following treatment. Additionally, one ought to arrange to remain a short drive from the site of the procedure. Additionally, for two months following treatment, one should arrange to have someone drive you where you need to go.
While CAR T-cell therapy has demonstrated remarkable efficacy in treating certain cancers that are difficult to treat, it can occasionally result in severe or even fatal side effects. As a result, it must be administered in a hospital with specialized training in its use, and patients must be closely monitored for a few weeks following their administration of CAR T cells.
Cytokine release syndrome (CRS): As CAR T cells proliferate, they may discharge copious quantities of substances known as cytokines into the bloodstream, thereby enhancing the immune system. This release may cause serious side effects, which include:
Physicians are learning how to identify CRS early and how to treat it as they become more experienced with CAR T-cell therapy.
Nervous system issues: This medication may occasionally have detrimental effects on the nervous system, which may cause symptoms like:
Adult patients are usually advised not to drive, operate heavy machinery, or engage in any other potentially dangerous activities for at least several weeks following treatment due to the possibility of these side effects.
Other detrimental effects: The following are additional major adverse effects of CAR T-cell therapy:
One must notify the doctor of any side effects as soon as possible for those who are receiving CAR T-cell therapy, as medications can frequently be used to treat them.
CAR T cells are analogous to “giving patients a living drug.”
The foundation of CAR T-cell therapy is, as their name suggests, T cells, which aid in coordinating the immune response and specifically destroy pathogen-infected cells. The CAR T-cell treatments that are currently on the market are tailored to each patient specifically. To create them, T cells are extracted from the patient and modified in a lab to produce proteins on their surface known as chimeric antigen receptors, or CARs. Certain proteins, or antigens, on the surface of cancer cells are recognized and bound to by the CARs.
Connect with Best Oncology Hospitals
Get Free Personalized quote for CAR T Cell Therapy
 |
 |
 |
 |
 |
Many patients who have undergone CAR T cell therapy showed promising and exciting results. 80% of patients showed reduction or complete elimination of cancer cells after the therapy.
In cases of adults and children treated with Kymriah® therapy, 80% of adults and children with ALL showed remission (recovery period).
This therapy has shown effective results in the challenging cases where the standard cancer treatments – chemotherapy and radiation failed.
The FDA has approved CAR T cell therapy for patients who have certain types of leukemia or lymphoma that has relapsed/recurred or progressed even after other treatments.
To be eligible for the CAR T cell treatment, the patient must have a history of unsuccessful treatment with least two standard cancer therapies.
Children and adults of up to 25 years of age with advanced and relapsed or refractory acute lymphoblastic leukemia (ALL) are approved for Kymriah® therapy.
Yescarta® is approved for adults with large B-cell non-Hodgkin lymphomas which is aggressive and recurring or has not been fully eradicated after at least two prior cancer therapies.


Cost Calculator

Top Specialities
Top Treatments
Best Hospitals for Top Treatments
Latest Articles
Disclaimer: Lyfboat does not provide professional medical opinion on the treatment or diagnosis of a particular ailment. All the offered services and information presented on www.lyfboat.com are only for the purpose of public knowledge and cannot substitute the professional consultation of the physician. Lyfboat strongly advice against copying or cloning of its web content and follows the legal protocols for protection of its intellectual property.
© 2024 Lyfboat Technologies Pvt. Ltd. All Rights Reserved.
Please wait